- All
- Product Name
- Product Keyword
- Product Model
- Product Summary
- Product Description
- Multi Field Search

loading
Goldsite
| Availability: | |
|---|---|

GT-100 SARS-CoV-2 Neutralizing Antibody Kit uses advanced immunofluorescence-based lateral flow technology for detection of neutralizing antibodies (NAb) to SARS-CoV-2 in human whole blood, serum and plasma. The GT-100 SARS-CoV-2 Neutralizing Antibody Kit, with the GT-100 Fluorescence Immunoassay Analyzer(FIA), provides automated and objective results in 12 minutes, allowing for monitoring immunity response after the second dose of the COVID-19 vaccine in point-of-care testing(POCT) scenarios. |
Measuring the neutralizing antibodies potency are expected to build a defense against SARS-CoV-2 spreading, complementing vaccines and nonpharmaceutical interventions.
Understanding the human body’s immune response to SARS-CoV-2, the virus that causes COVID-19, is key to developing effective treatments and long-lasting vaccines.
Potent nAbs that were more effective at neutralizing the virus have better clinical outcomes. Early administration of nAbs is vital to identify patient of severity risk as an aid to treatment.
Only 12 mins for results.Suitable for large scale testing in point-of-care (POCT) way.
Up to 100% positive agreement with gold standard VNT method.
Simple test workflow, labor saving.
Objective result that eliminates the subjectivity of a visual read.
| Cat. | GT12403 |
| Description | GT-100 SARS-CoV-2 Neutralizing Antibody(NAb) Kit |
| Package size | 25T/Box |
| Sample Type | whole blood, plasma, serum |
| Cat. | GT12401 |
| Description | GT-100 SARS-CoV-2 IgM/IgG Antibody(Ab) Kit |
| Package size | 25T/Box |
| Sample Type | whole blood, plasma, serum |
| Cat. | GT12401 |
| Description | GT-100 SARS-CoV-2 Antigen(Ag) Kit |
| Package size | 25T/Box |
| Sample Type | Nasal swab |

GT-100 SARS-CoV-2 Neutralizing Antibody Kit uses advanced immunofluorescence-based lateral flow technology for detection of neutralizing antibodies (NAb) to SARS-CoV-2 in human whole blood, serum and plasma. The GT-100 SARS-CoV-2 Neutralizing Antibody Kit, with the GT-100 Fluorescence Immunoassay Analyzer(FIA), provides automated and objective results in 12 minutes, allowing for monitoring immunity response after the second dose of the COVID-19 vaccine in point-of-care testing(POCT) scenarios. |
Measuring the neutralizing antibodies potency are expected to build a defense against SARS-CoV-2 spreading, complementing vaccines and nonpharmaceutical interventions.
Understanding the human body’s immune response to SARS-CoV-2, the virus that causes COVID-19, is key to developing effective treatments and long-lasting vaccines.
Potent nAbs that were more effective at neutralizing the virus have better clinical outcomes. Early administration of nAbs is vital to identify patient of severity risk as an aid to treatment.
Only 12 mins for results.Suitable for large scale testing in point-of-care (POCT) way.
Up to 100% positive agreement with gold standard VNT method.
Simple test workflow, labor saving.
Objective result that eliminates the subjectivity of a visual read.

| Cat. | GT12403 |
| Description | GT-100 SARS-CoV-2 Neutralizing Antibody(NAb) Kit |
| Package size | 25T/Box |
| Sample Type | whole blood, plasma, serum |
| Cat. | GT12401 |
| Description | GT-100 SARS-CoV-2 IgM/IgG Antibody(Ab) Kit |
| Package size | 25T/Box |
| Sample Type | whole blood, plasma, serum |
| Cat. | GT12401 |
| Description | GT-100 SARS-CoV-2 Antigen(Ag) Kit |
| Package size | 25T/Box |
| Sample Type | Nasal swab |

GT-100 adopts time-resolved fuorescence immunoassay technology and uses europium, a rare lanthanide element, with long fuorescence lifetime and wide detection window
Excitation wavelength 340nm, emission wavelength 613nm, narrow emission peak, wide Stokes shift (wavelength difference between emission and excitation spectrum)
Easy to distinguish non-specific (background) fluorescence interference between excitation spectrum and emission spectrum, and fast disappearance of background fluorescence.
Dissociation enhancement technology can improve the fuorescence signal by 1 million times, and the detection accuracy can reach 10 mol/L.
Principle of time-resolved fuorescence immunochromatography
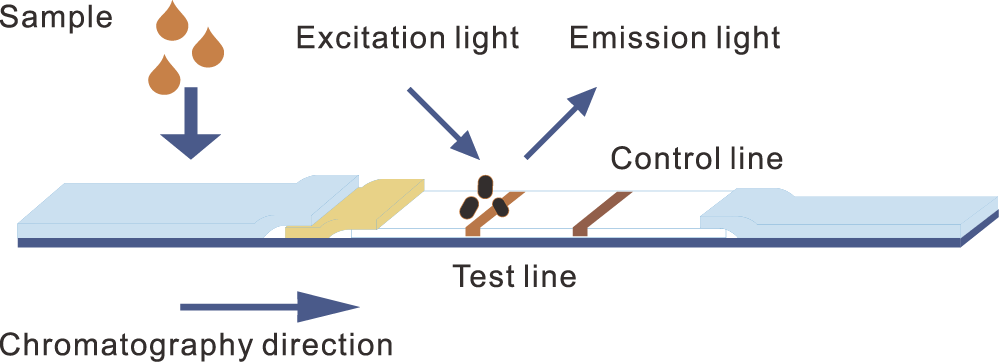
Principle of time-resolved fluorescence technique
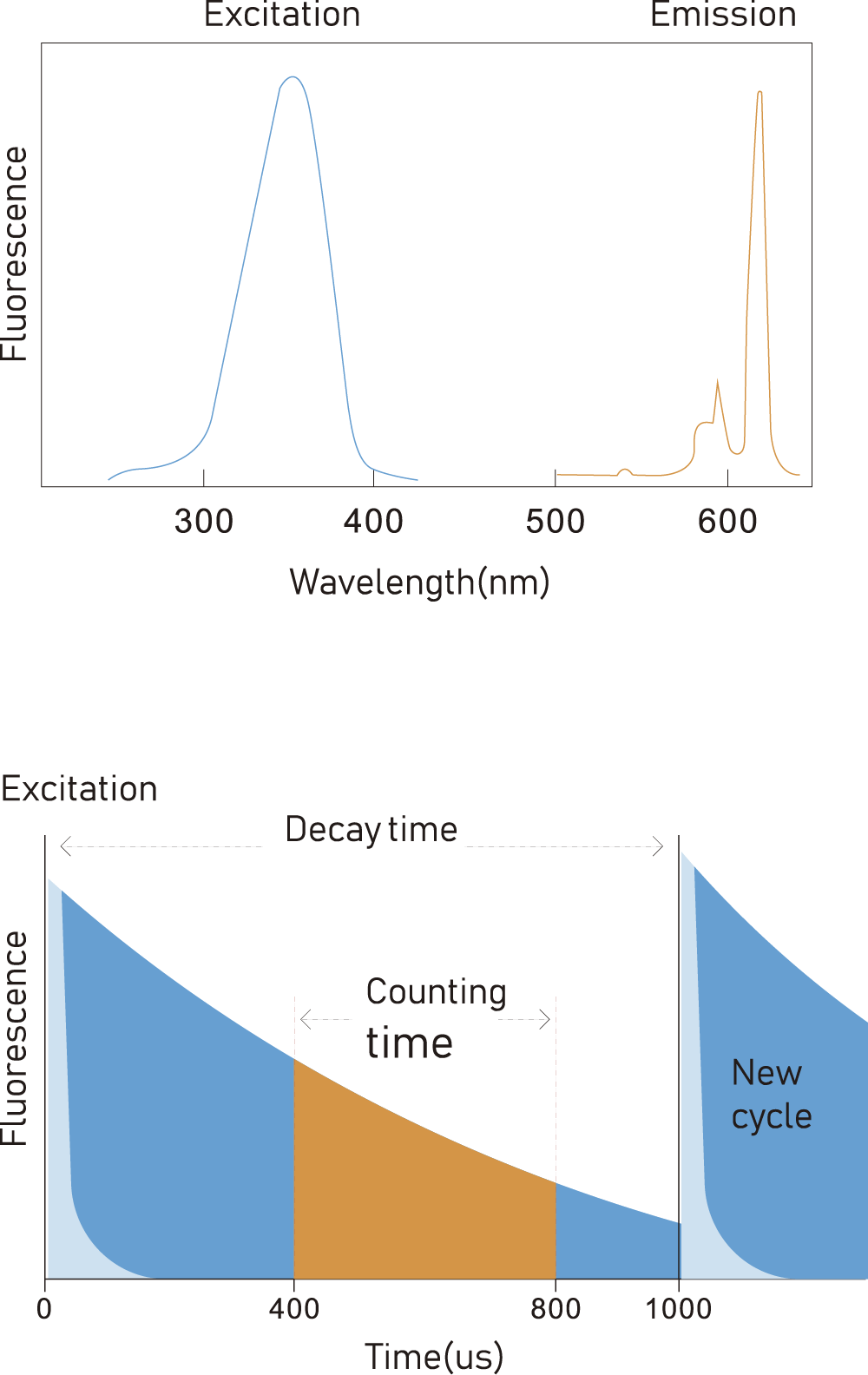
Time-resolved fluorescence immunoassay, high precision, low interference;
Insert card to import calibration curve and reagent information,
Disposable reagent card, avoid cross-contamination
One-step operation, 20 seconds to issue the test report;
Whole blood can be added directly to the test, no manual dilution;
Test results are automatically uploaded to LIS system, bitin printer
Microvolume blood needed as low as 2μL;
Support for venous / peripheral whole blood, serum, plasma samples;
| Cassette type | Strip type |
 |  |
| Assay | Cartridge Type | Clinical significance |
|---|---|---|
| SARS-CoV-2 Antigen(Ag) | Strip | Rapid screening of suspected COVID-19 cases |
| SARS-CoV-2 Neutralizing Antibody(NAb) | Strip | Monitoring COVID-19 vaccine efficacy |
| SARS-CoV-2 IgM/IgG Antibody(Ab) | Strip | Aid testing for RT-PCR negative or suspected cases |
CRP+SAA | Cassette | Infection diagnosis and differentiation of infection types |
cTnl | Strip | Myocardial injury markers |
NT-proBNP | Strip | Heart Failure Markers |
| cTnI+NT-proBNP | Cassette | Myocardial injury, heart failure |

GT-100 adopts time-resolved fuorescence immunoassay technology and uses europium, a rare lanthanide element, with long fuorescence lifetime and wide detection window
Excitation wavelength 340nm, emission wavelength 613nm, narrow emission peak, wide Stokes shift (wavelength difference between emission and excitation spectrum)
Easy to distinguish non-specific (background) fluorescence interference between excitation spectrum and emission spectrum, and fast disappearance of background fluorescence.
Dissociation enhancement technology can improve the fuorescence signal by 1 million times, and the detection accuracy can reach 10 mol/L.
Principle of time-resolved fuorescence immunochromatography

Principle of time-resolved fluorescence technique

Time-resolved fluorescence immunoassay, high precision, low interference;
Insert card to import calibration curve and reagent information,
Disposable reagent card, avoid cross-contamination
One-step operation, 20 seconds to issue the test report;
Whole blood can be added directly to the test, no manual dilution;
Test results are automatically uploaded to LIS system, bitin printer
Microvolume blood needed as low as 2μL;
Support for venous / peripheral whole blood, serum, plasma samples;

| Cassette type | Strip type |
 |  |
| Assay | Cartridge Type | Clinical significance |
|---|---|---|
| SARS-CoV-2 Antigen(Ag) | Strip | Rapid screening of suspected COVID-19 cases |
| SARS-CoV-2 Neutralizing Antibody(NAb) | Strip | Monitoring COVID-19 vaccine efficacy |
| SARS-CoV-2 IgM/IgG Antibody(Ab) | Strip | Aid testing for RT-PCR negative or suspected cases |
CRP+SAA | Cassette | Infection diagnosis and differentiation of infection types |
cTnl | Strip | Myocardial injury markers |
NT-proBNP | Strip | Heart Failure Markers |
| cTnI+NT-proBNP | Cassette | Myocardial injury, heart failure |
We offer